Transcribed Image Text from this Question
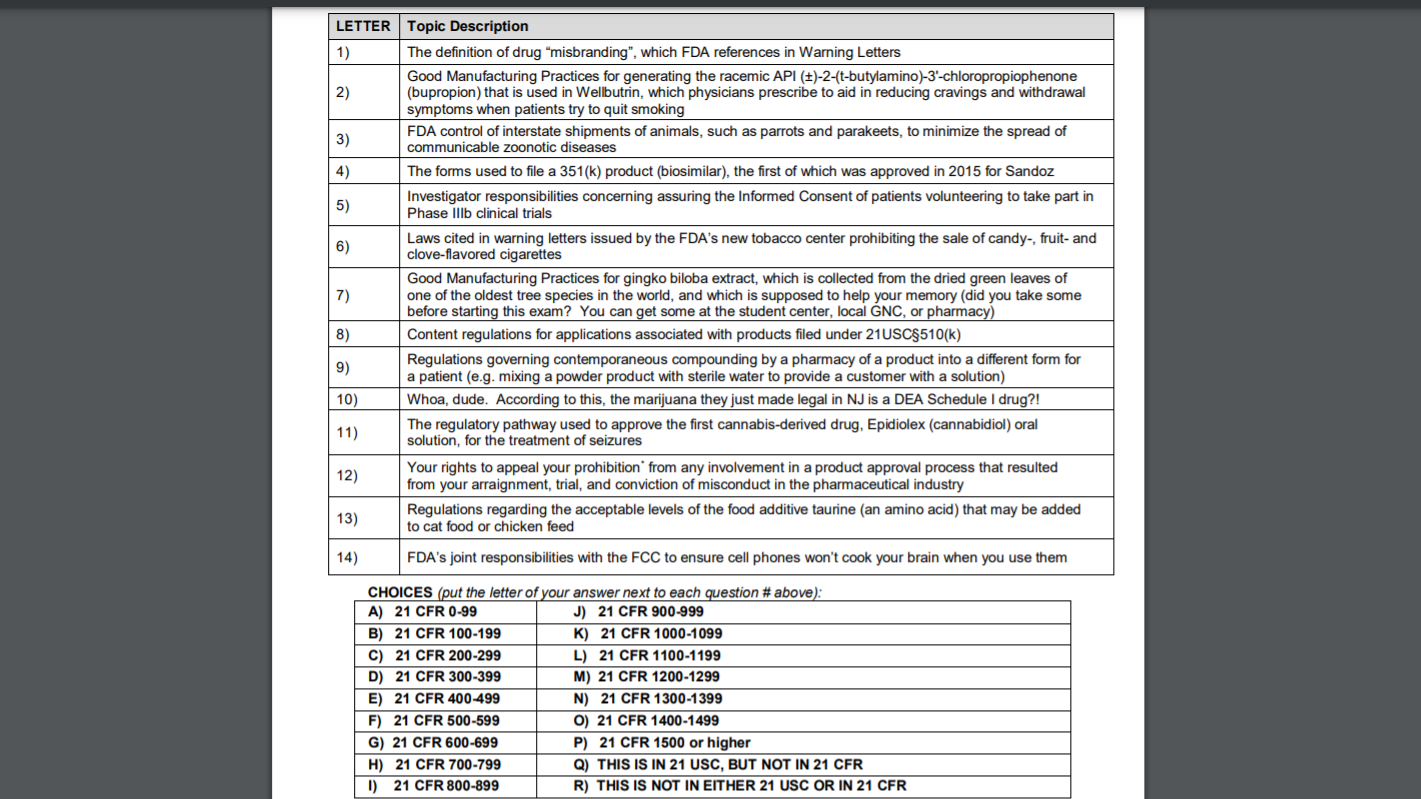
LETTER 1) 2) 3) 4) 5) 6) 7) Topic Description The definition of drug “misbranding”, which FDA references in Warning Letters Good Manufacturing Practices for generating the racemic API (+)-2-(t-butylamino)-3′-chloropropiophenone (bupropion) that is used in Wellbutrin, which physicians prescribe to aid in reducing cravings and withdrawal symptoms when patients try to quit smoking FDA control of interstate shipments of animals, such as parrots and parakeets, to minimize the spread of communicable zoonotic diseases The forms used to file a 351(k) product (biosimilar), the first of which was approved in 2015 for Sandoz Investigator responsibilities concerning assuring the informed Consent of patients volunteering to take part in Phase IIIb clinical trials Laws cited in warning letters issued by the FDA’s new tobacco center prohibiting the sale of candy-, fruit- and clove-flavored cigarettes Good Manufacturing Practices for gingko biloba extract, which is collected from the dried green leaves of one of the oldest tree species in the world, and which is supposed to help your memory (did you take some before starting this exam? You can get some at the student center, local GNC, or pharmacy) Content regulations for applications associated with products filed under 21USC3510(k) Regulations governing contemporaneous compounding by a pharmacy of a product into a different form for a patient (e.g. mixing a powder product with sterile water to provide a customer with a solution) Whoa, dude. According to this, the marijuana they just made legal in NJ is a DEA Schedule I drug?! The regulatory pathway used to approve the first cannabis-derived drug, Epidiolex (cannabidiol) oral solution, for the treatment of seizures Your rights to appeal your prohibition from any involvement in a product approval process that resulted from your arraignment, trial, and conviction of misconduct in the pharmaceutical industry Regulations regarding the acceptable levels of the food additive taurine (an amino acid) that may be added to cat food or chicken feed FDA’s joint responsibilities with the FCC to ensure cell phones won’t cook your brain when you use them 8) 9) 10) 11) 12) 13) 14) CHOICES (put the letter of your answer next to each question # above): A) 21 CFR 0-99 J) 21 CFR 900-999 B) 21 CFR 100-199 K) 21 CFR 1000-1099 C) 21 CFR 200-299 L) 21 CFR 1100-1199 D) 21 CFR 300-399 M) 21 CFR 1200-1299 E) 21 CFR 400-499 N) 21 CFR 1300-1399 F) 21 CFR 500-599 O) 21 CFR 1400-1499 G) 21 CFR 600-699 P) 21 CFR 1500 or higher H) 21 CFR 700-799 Q) THIS IS IN 21 USC, BUT NOT IN 21 CFR 1) 21 CFR 800-899 R) THIS IS NOT IN EITHER 21 USC OR IN 21 CFR
(Visited 5 times, 1 visits today)