Transcribed Image Text from this Question
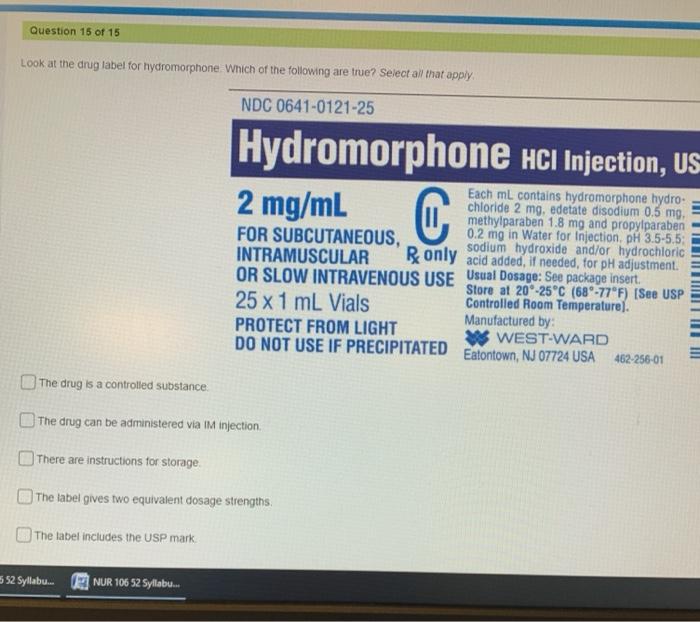
Question 15 of 15 Look at the drug label for hydromorphone. Which of the following are true? Select all that apply NDC 0641-0121-25 Hydromorphone HCl Injection, US 2 mg/mL C Each mL contains hydromorphone hydro- chloride 2 mg, edetate disodium 0.5 mg, methylparaben 1.8 mg and propylparaben FOR SUBCUTANEOUS, 0.2 mg in Water for Injection, pH 3.5-5.5: sodium hydroxide and/or hydrochloric INTRAMUSCULAR Bonly acid added, if needed for pH adjustment. OR SLOW INTRAVENOUS USE Usual Dosage: See package insert Store at 20-25°C (680-77°F) [See USP 25 x 1 ml Vials Controlled Room Temperature). Manufactured by: PROTECT FROM LIGHT WEST-WARD DO NOT USE IF PRECIPITATED Eatontown, NJ 07724 USA 462-256-01 III III III The drug is a controlled substance The drug can be administered via IM injection There are instructions for storage The label gives two equivalent dosage strengths The label includes the USP mark 552 Syllabu. NUR 10652 Syllabu…
(Visited 6 times, 1 visits today)



