Transcribed Image Text from this Question
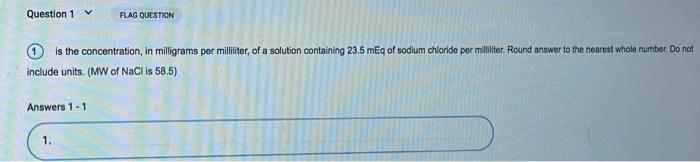
Question 1 FLAG QUESTION is the concentration, in milligrams per milliliter, of a solution containing 23.5 mg of sodium chloride per milliliter. Round answer to the nearest whole number. Do not include units. (MW of NaCl is 58,5) Answers 1 – 1 1.
(Visited 3 times, 1 visits today)
Related posts:
- Question: Question 5 L FLAG QUESTION 1 Grams Of Sodium Chloride (MW Of NaCl Is 58.5) Should Be Used To Prepare 220 ML Of A Sodium Chloride Solution Containing 3 MEq Na Per ML. Round To The Nearest Tenth. Do Not Include Units.
- Question: Question 3 FLAG QUESTION Trace Electrolytes Additive (Tracelyte) Contains 0.25 MEq Of Calcium Per Milliliter. If 20 ML Of Tracelyte Is Included In 1 L Of TPN Solution, This Would Be Equivalent To 1 Milligrams Of Calcium Chloride (molecular Weight Of CaCl, = 111). Round Answer To The Nearest Tenth. Do Not Include Units.
- Question: Question 9 V FLAG QUESTION 1 Grams Of Potassium Citrate Monohydrate Powder Are Needed To Prepare 1 L Of An Oral Solution Containing 2mEq Of Potassium Per Milliliter? (molecular Weight Of K_CHO_.H2O = 324). Round Answer To Nearest Whole Number. Do Not Include Units. Answers 1 – 1
- Question: Question 2 FLAG QUESTION . A Solution Contains 5% Dextrose And 0.45% Sodium Chloride. The Osmolarity Of The Solution Is Molecular Weight Of Dextrose = 180, Molecular Weight Of NaCl = 58.5. Round Answer To The Nearest Tenth Do Not Include Units. Answers 1-1
- Question: Question 2 FLAG QUESTION A Solution Contains 5% Dextrose And 0.45% Sodium Chloride. The Osmolarity Of The Solution Is 1 Molecular Weight Of Dextrose = 180, Molecular Weight Of NaCl = 58.5. Round Answer To The Nearest Tenth. Do Not Include Units. Answers 1-1
- Question: Question 23 FLAG QUESTION 1 Is The Osmolarity Of 1000 ML Of 20% W/v Dextrose And 0.45% W/v Sodium Chloride Containing 12 MEq Of Magnesium Sulfate. Round Answer To The Nearest Hundredth. Do Not Include Units. (MW Of Dextrose = 180; MW Of NaCl = 58.5 And MW Of MgSO, = 120)
- Question: Question 8 FLAG QUESTION K-TAB Tablets Contain 20 MEq Of Potassium In The Form Of Potassium Chloride 1 Is Milligrams Of Potassium Chloride In Each Tablet. Round To The Nearest Whole Number. Do Not Include Units. (MW Of KCl Is 74.5) Answers 1-1
- Question: Question 17 FLAG QUESTION … … How Much 1 : 25 Solution And 1:500 Solution Should You Mix To Make 1 L Of A 1 : 250 Soaking Solution? Answers Are Rounded To The Nearest Whole Number. Answers A-D A 750 ML Of The 1 : 25 Solution And 250 ML Of The 1: 500 Solution B 250 ML Of The 1: 25 Solution And 750 Of The 1 : 500 Solution с 53 ML Of The 1 : 25 Solution …
- Question: Question 20 FLAG QUESTION If A Pharmacist Wished To Prepare 100 ML Of A Solution Containing 50 MOsmol Of Calcium Chloride (MW Of CaCl = 111) , Grams Of Calcium Chloride Would Be Needed. Round To The Nearest Hundredth. Do Not Include Units. Assume Complete Dissociation. Answers 1-1
- Question: Question 22 V FLAG QUESTION Half-normal Saline Is 0.45% Sodium Chloride. 1 Is Its Concentration In MEq/mL (MW Of NaCl Is 58.5). Round Answer To The Nearest Tenth. Do Not Include Units. Answers 1-1



