Please read the Tay (2017) article from Diabetes, Obesity, andMetabolism (picture attach below) to answer the followingquestions.
Questions:
1) What kind of study design was used in the Tay (2017)paper?
a.What were the specific treatment conditions?
b. What was the primary outcome?
c.What was the follow-up period?
2) In what ways does this kind of study provide morevalidity than an observational design?
3) Which treatment group showed the best results interms of the primary outcome?
4) Based on your answer to #3, which source of energyseems to be the greatest risk factor for type 2 diabetes diseaseprogression?
Picture of the Tay (2017) article:
Transcribed Image Text from this Question
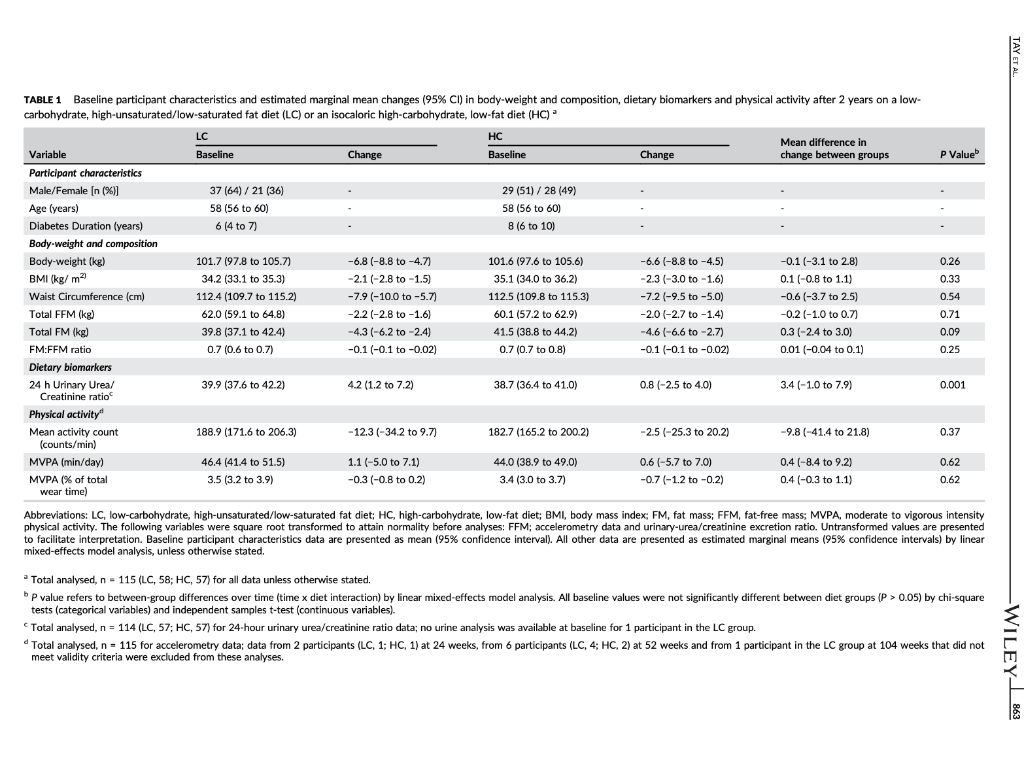
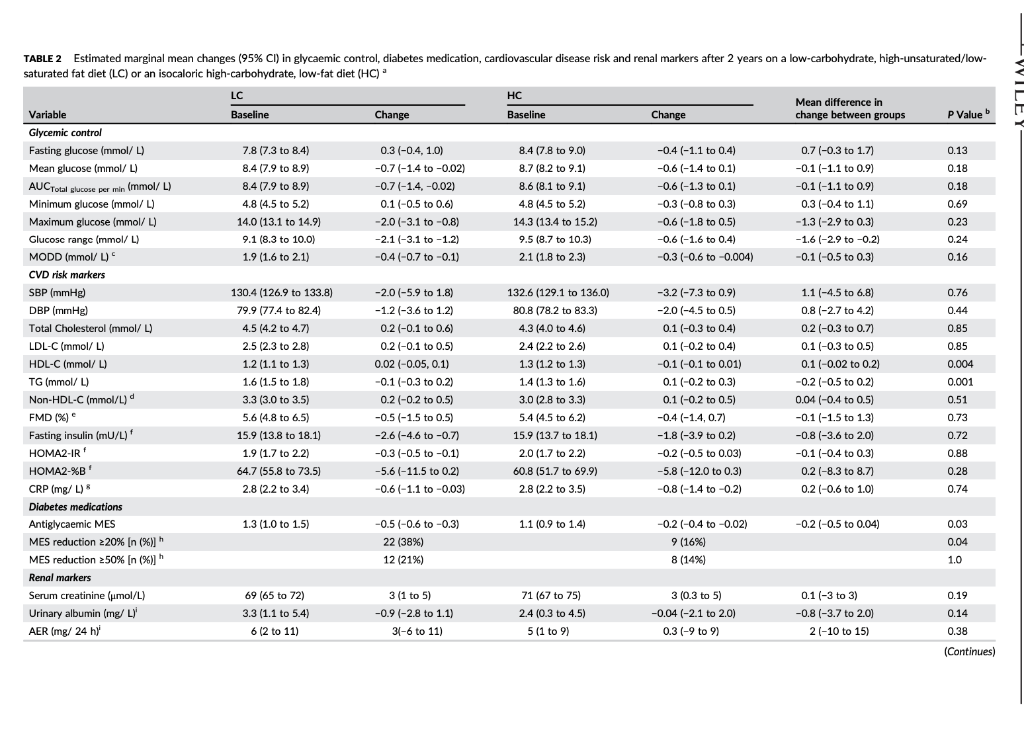
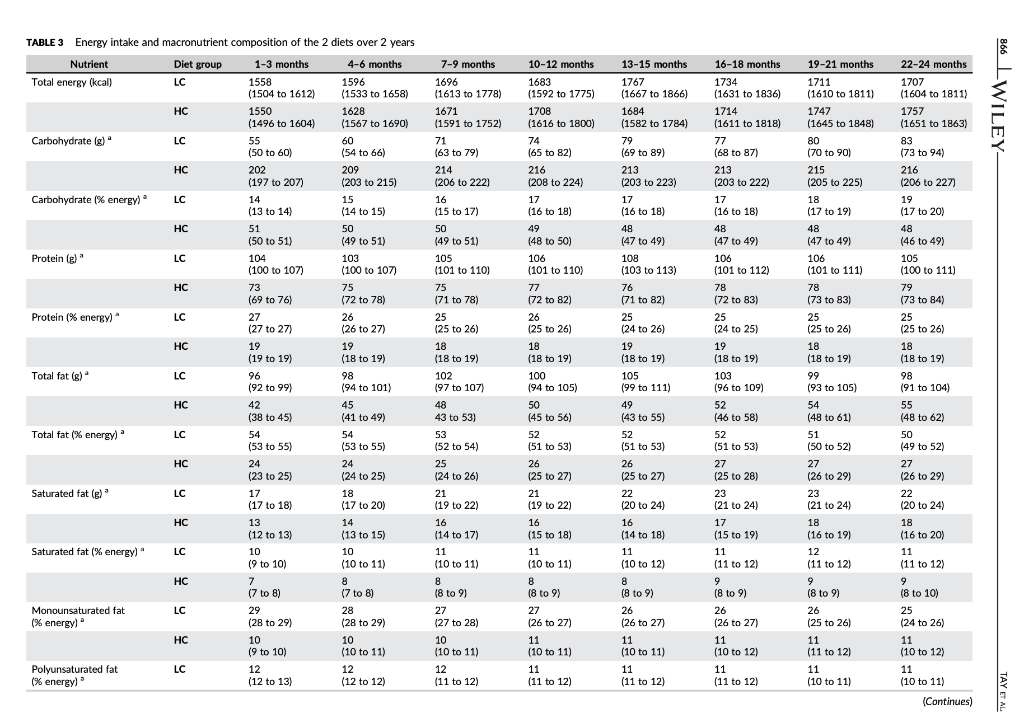
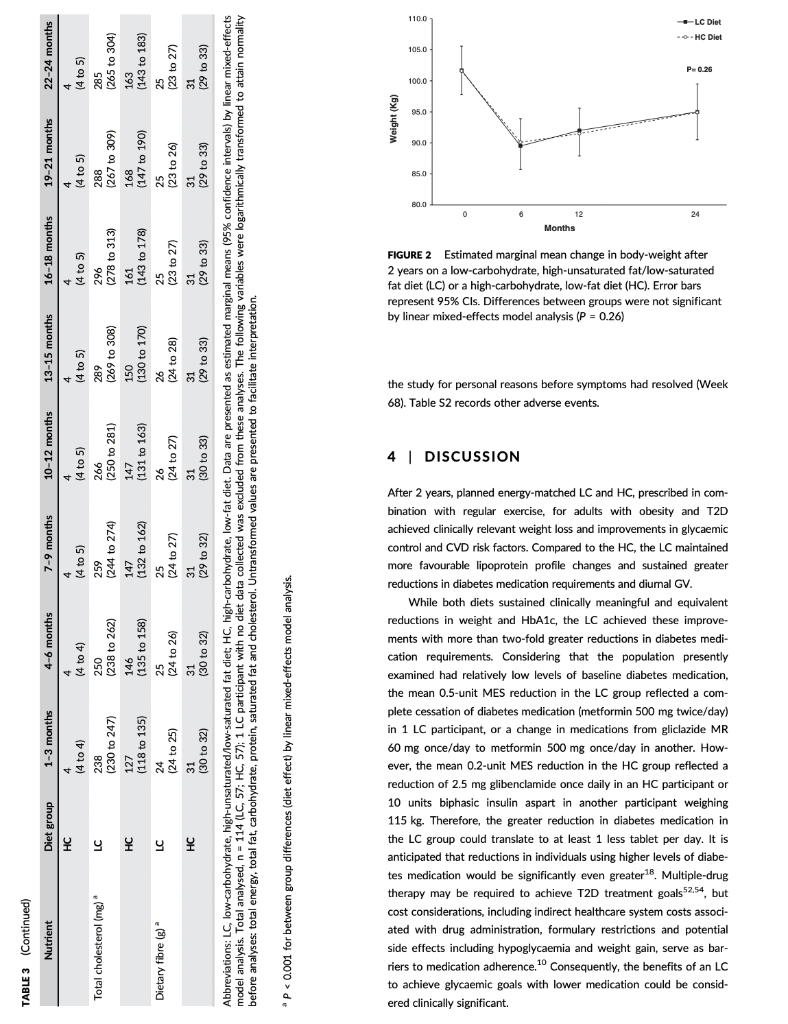
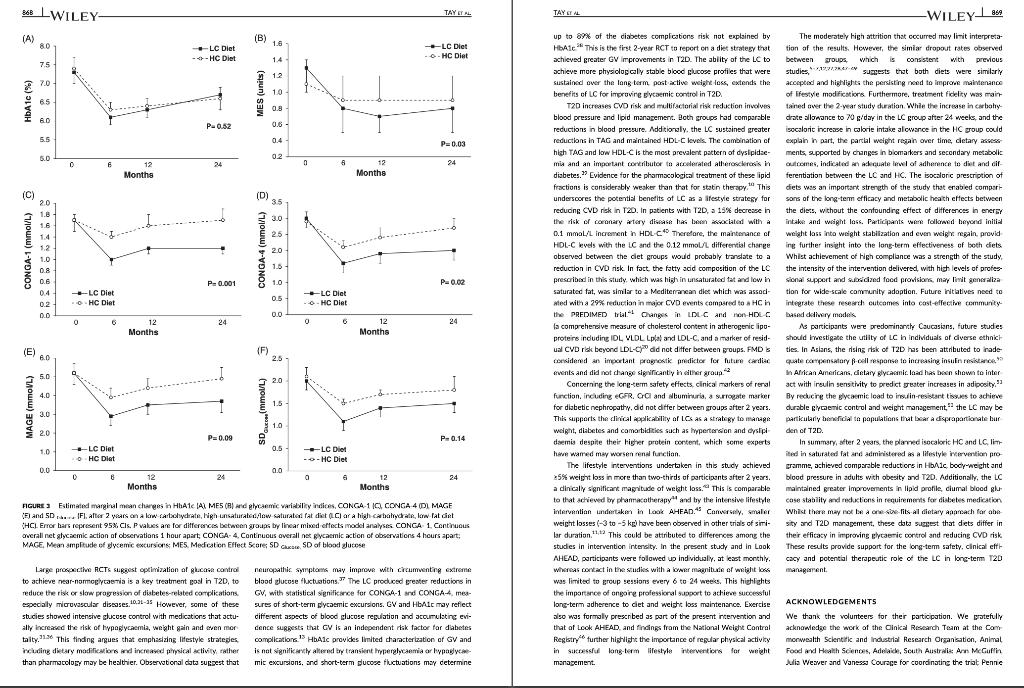
TAYT Received: 13 August 2017 | Roxtel. 24 October 2017 Accepted: 14 November 2017 . | Accepoe DOI: 10.1111/dom. 13164 -WILEY 259 1 | INTRODUCTION WILEY comprehensive evaluation of Rycaemic control, anthropometry and CVD risk markers in obese adults with T2D. ORIGINAL ARTICLE 2 MATERIALS AND METHODS Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high- carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial Jeannie Tay PhD 1.2,6 Campbell H. Thompson MD² | Natalie D. Luscombe-Marsh PhD || Thomas P. Wycherley PhD? | Manny Noakes PhD? | Jonathan D. Buckley PhD || Gary A. Wittert MD? | William S. Yancy Jr MD45 | Grant D. Brinkworth PhD? The worldwide prevence of iv 2 diabetes T2D continues to surge despite therapeutic advances, highlighting the urgent need for more effective treatment strategies Lifestyle management encom passing nutrition therapy and physical activity for the camerstone of diabetes care. However, the most efficacious long-term nutrition therapy remains controversial. Whle leading health authorities now advocate an individualized dietary proach to dabetes management, diferent diets may vary in their efficacy in improving cycaemic control and reducing the risk of cardiovascular dis- case CVD. Low-fat, high-unrefined carbohydrate diets have been the pre- dominant public health weight-management recommendation for the past several decades and have typically been prescribed for the die tary management of T2D.” However, emergre evidence suggests that carbohydraterestriction and higher intakes of protein and unsat urated fats, independent of weight loss, improve glycaemic control and some CVD risk markers, potentially conferring greater benefits over high-carbohydrate diets-Hyperglycaemia is a salient charac teristic of T2D, and dietary carbohydrates, particularly those that are refined, are the greatest determinant of postprandial Dycaemia Restricting the intake of carbohydrates to alleviate hyperglycaemia can lead to fewer glycaemic excursions and alow for reduction of meditation Is this easily understood byd acceptableta 2.1 Study design and participants The study design has been described previously 15.06 This outpatient single-centre, parallel-groups. RCT was conducted from May 2012 through Septembar 2014 at the Commonwealth Scientific Industrial Research Organisation ICSIRO Clinical Research Unit (Adelaide, Australia. Partidpants with established T2D under the care of a gen er practitioner and/or endocrinologist were recruited from the com- munity, primarily through media advertisements, and included individuals aged 3 to 6 years with T2D HbAlc 2 7.0% and/or using diabetes medication including insulirt and with body mass index (BMI) of 26 to 45 kg’m. Major exclusion criteria were: type-1 diabetes, real hepatic, respiratory, gastrointestinal or cardiovascular disease; history of malignancy: any significant endocrinopathy other than stable treated thyroid cisease pregnancy/bctation: History of ar current entre disorder; ar smaking. All study parints provided written informed consent The CSIRO Human Research Ethics Com mittee approved the study. Peticipants were bock-matched for ay gender, EMI, HbA1c and diabetes medication using random varying block stres and were allocated to the LC or HC (1:1) by random computer generated sssignment Figure 1). The research associates who conducted these randomization procedures were not involved in outcome assements and intervention delivery. The researders involved in outcome sessment and data analysis were blinded to treatment assignment Commonwealth Sciend and Industrial Research On ICS ROI – Health and Biosecur Ly, Adlaide Australa Adaripe Medicine University of Adele Atletisie Antw Niance for Research in Exerdse. Nutrition and Acthay (ARENAS rute for Health Research University of South Austria Adele, Australia Division of General Trul Medicine, Department of Mecken Dure University Medical Center, Duta, North Carina Center for Health Services Research in Primary Care Veterans Affairs Medical Center the North Carolina Agency for why ASIA Spor Correspondence Grant Birworth, PhD, Connorwath Scientific and Industriech Organization Health and Bivaccurly Riverside Corporate Park 11 Julius Ann. North NSW, Austria 2113 Email: arikerho Funding Information This study was supported by Nationalth and Medical Research Council of Australia Project Grant 10151 T. was poored by a postigadiste research scholarship from the Agency for Science, Tedinology and Research (A-STAR. Singapore Fuidig sponsors role in the design. conduct or reporting of the study or creacion to the maruwari for publication Aim: To examine whether a low-carbohydrate, high-unsaturated/low-saturated fat diet IL) Improves glycaemic control and cardiovascular disease (CVD risk factors in overweight and play with type 2 diabetes 20. Methods: A total of 115 adults with T2D (rean (SD): BMI 346 1.30 kgm?: age, 50 years: HbA1C, 7.3 (1.11%) were randomized to 1 of 2 planned energy matched, hypocaloric ciets com- bined with rot/resistance (1 hour, 3 days/weak for 2 years: LC: 14% energy carbohydrate, 20% as protein, 58% as fat (<10% saturated fatk or low-fat, high-carbohydrate, low lycaemic index diet (HC): 53% CHO, 17% as protein, 30% as tat (<10% saturated fat H41. glycaemic wariability (GM. anti-gycemic medication effect score (MES taidated based on the potency and dosape of diabetes medication weight, body composition. CVD and renal risk markers were assessed before and after intervention Results: A total of 61 (LC-33 HC – 28 participants completed the study trial registration: http://www.ancr.org.au/, ANZCTR NO ACTRN12612000369820). Reductions in weight (esti- mated marginal mean 95% CILLC -6.8|-&R-47 HC -6.6-88, -45 k), loody fat (LC-43 (-62.-24. HC, -461-66 -2.73 kpl blood pressure (LC.-20[-59, 181-12 1-3.6, 121, HC. -3.2 [-7.3, 0.94 -20 1-45,0 mmHg, HbAlc LC-06 :-0.9,-0.2, HC-0.9 [-1.2-0.5%) and tasting glucose ILC, 0.31-04, 101, HC, -041-11,04 mmol/L) were similar between groups P 2009). Compared to HC, the LC achieved greater reductions in diabetes medication use IMES: LC-05 1-0.6.-0.31. HC-02-04,-0.02 unit: P-0031. GV Continuous Overal Net Glycemic Action calculated every 1 hour (LC, -04 (-0.6 -0.3] HC, -0.1 (-0.1, 02 mmol/l: P-00011 and 4 hours (LC.-09|-13-OL HC-02-06, 0.1 mmol/L-00211; Luce ides (LC.-01 (-0.3.021, HC. 01 (-0.2.0.3 mmoll: P-0001), and maintained HOL-C levels ILC, 0.02 -0.05 0.1], HC, -0.1 (-0.1, 0.01) mmol/L; P=0.0041, but had similar changes in LDL- CILC,02 -0.1, 0.51 HC, 01-02.04 mall: P-0.5.bchid artery flow mediated data- tion LC.-0.5-15.O.SI, HC-04 1-14,0.71 %P-0.79) EGFR and albuminuria. Conclusions. Both diets achieved comparable weight loss and HbAlc reductions. The LC sus tsinec greater rech.ctions in diabetes medication requirements, and in improvements in diurnal blood glucose stability and blood lipid profile with no adverse renal effects. qucpesting greater optimization of T20 management Despite the greater interest in and use of low-carbohydrate diets. their long-term effectiveness and sustainability in Individuals with T2D have not been well sed. Current guidelines assert that there is insufficient evidence in isccaloric comparisons to recommend an ideal carbohydrate intake or to recommend such diets, over other diets for individuals with diabetes. 1.10 Amores the limited number of studies of low-carbohydrate diets in individuals with T20 beyond 1 year, 1 study prescribed a relatively high carbohydrate composition -150-189/day. 40% energy in the low-carbohydrate ciert group and included only a small subgroup of 36 people with T2D.” Another study administered a low intensity intervention with limited prales sional contact that resulted in reduced treatment adherence 12 Nei- ther study controled for differences in energy intakes, assessed charm in diabetes medications or glycemic variety ICV. emerging as an independent risk factor for diabetes complications, nor considered physical activity. To address these limitations, we designed a randomized controlled trial RCT aimed at comparing the effectiveness of 2 isocaloric diets in individuals with T20: 3 low- cartchydrate and low saturated far diet (LCI VS a conventional low fat, higher-carbohydrate low-plycemic index dies HC. We previously reported that over 1 year, the LC produced greater improvements than the HC in camic controllower diabe tes medication requirements and GVI. and more favourable lipid pro- file changes increased HDL-C and reduced triglycerides (TG), in chills with T20. We reported these will given their clinical importance to this high-risk study population We now report the longer term 12-year stability of these effects by comparing isocaloric LC and HC as part of a Ffestyle inter- vention incorporating structured ise regime, with a 2.2 | Diet and physical activity interventions The planned macronutrient compositions of the 2 ciets were: LC, 14% ceboldatei 50 / 28%, protein and 5% total fat (35% monounsaturated fat and 13% polyunsaturated fatl. with the inclus sion of an additional 20 g carbohydrate alowance after week 24 for the remainder of the study, HC, 53% carbohydrate processed carbo- hycrates and high glycaemis Index foods were discourage, with an emphasis on the selection of low glycaemic foods; Overall glycaemi index of 461, 17% protein and <30% total fat (15% monounsaturated tot and 9% polyunsaturated fat, reflecting traditional dictary guide fines with the indusion of an approved food exchange with the macronutrient profile of the diet and was equivalent to the energy content of 20g of carbohydrate) after week 24 for the remainder of the sturly so that the dots remained vari. Satu- rated fat was imited in both diets (< 10% energy! Participants met individually with a detition for diet instruction and support every 2 weeks for 12 weeks and monthly thereafter During the line 12 weks participants were provided with key foods 1-30% total energy representative of their assigned diets to achieve the targeted macronutrient profiles (Table 51). These foods were isted in semi-cutiative food record that participants completed daily. After 12 weeks, for the remainder of the study, participants KEYWORDS diecary intervention, type 2 diabetes, weight control 858 2017 John Wiley & Sort Lod Wiconlindrer roumai com Diabetes Obes Mccul. 2015 20.858 87. 10 LWILEY HI TAYPTM. TAYT -WILEY Reperen X-ray absorptiometry IDEXA Lunar Prodigy: General Electric Corpo ration, Madison, Wisconsin Quis rexived Screened (200) 2143 kcal/day energy prescription. Calorit prescriptions were maintained throughout the study to preserve planned Isocaloric con trol between ciets. The same professionally supervised 60-minute exerdse classes, incorporating moderate intensity aerobic ad resistance exercise on 3 non-consecutive days per week were prescribed for al participants The dietitians and exercise professional responsible for delivering the intervention were trained in behavioural strategies, including motivational Interviewing and goal setting techniques that were Applied during the intervention. This rew.ch design enabled the cffects of the diets to be studied in the context of lifestyle interven- tion, whilst maintaining the ability to address the a priori research objective of comparing and isolating the differential effects of the HC and LC diets on the outcomes. wilidurs பார் பால்யவisu (5) Bible bar park 16 در ادامه، با اما 2.5.3 Cardiovascular and metabolic measures Resting load pressure was sured by Automated spreme nometry (SureSigns VS Philips. Andover, Massacusetts). Plasma glu- cose, serum total cholesterol HDL-CTG and CRP were measured on a Roche Hitachi 902 auto-analyser (Hitachi Science Systems Ltd, bar- al Japan) using standard enzymatic lits Roche Diagnostics, Indarapais, Indiana. LDL vil www cakulted using the Friede wall ecuation Non-HOL-C was calculated as the difference between total cholesterol and HDL C. Plasma insulin concentra tions were determined using a commercial enzyme immunoassay kt Mercoda AB Uppsala, Sweden. HOMA Index 2sessed cell func- Lion HOMA-YA1 and insulin manca HOMA-IRI”. Flora mediste vasodilatation (FMDI of the brachial artery was evaluated according to recommended guidelines, as previously described.22 Enrollment Withdrawals prior to treat groep dcloure my Heat (109 Withdrawals pelor to treatment group disceral stuals IEEEERAI பாலி) Immand in-> Tius: Un [u=21 Work Time cutie in Lulullah Chapain) Commenced study (n=115) LC L.w.cartyrale, biglaan al dict Higdo-carbuday drake, -luk dict (135) 23 Outcome measures Body weight and plasma katones were measured monthly through the study. All other chata were collected baseline and 24,52 and 104 weeks. At each time point, fasting blood samples were collected from a foram van inte ta containing no additives for lipids, insu lin C-reactive protein (CRP) and creatinine: sodium fluoride/EDTA for glucose and ketones and potassVEDTA for HbA1c. Plasma ar serum was isolated by centrifugation at 2000 for 10 minutes at 50 Beckman GS-GR centrifuge: Brea, Califomia) and stored at -90C until lysed. Line camps to stealbumin were frozena-20C in polyethylene tubes until analysed . 254 Renal function markers Serum creatinine was measured on clinical analyser Beckman Augu: Veckman Coultering, Brea, Califomis using a standardized y (Beckman kit OSR61781. Glomerular filtration rate was est mated by the Chronic kidney Disease Epidemiology Collaboration expation GFR CKD-EPI Creatinine Clearance (CC) was est- mated by the Cockcroft-Gault and the Salazar-Corcoran equations Albuminscretion rate AERI dunyanin from 24 hour une samples were measured at a certified commercial laboratory 15A Pathology, Adelaide Australlal Assed af 6 months in 46 Divulius Iranical -12) LAS TE Work.com (2) Lrable to comply wider) And at 6 months in IT) Dicnniul realisil in-LI L. CART Work citer (22) Unable to comply with det Perwaal гала катті Health shty-2 24 Primary outcome HbAIC ISA Pathology: Adelaide, Australia was the primary outcome – An al | year in Bed treatment Follow-up Womens Pasal (1-2) Thulthermal man in 1 A lycar (371 rectand treatment (11) Lost Ital) Time cestrais ir 15 Wuku) sa topy whi (1) Bewe Health All) 25.5 Diet and physical activity data Dietery intake was used from a random sample of 7 conce days of daily weighed food records within every 14-day period, using Foodworks Professional Edition Version 7 Xyris Software 2012, Highese Hil. Australis to obtain average quarterly nutrient intake over 104 weeks. The 24-hour urinary-urea creatinine ratio (IMVSI was assessed wake of protein intake Plasme ketones IB-hydroxybutyrate) were assessed as a marker of reduced cartohy drate intake (RANBUT D. Hydroxybutyratekt; Antrim, UK). Phys tal activity levels were assessed using data from 7 consecutive days of triaxial accelerometry (GT3X + modet ActiGraph, Pensacola, Flor icbal, with pre-defined widely cutoflow including mercise Session attendance And at 2 yun Disranned framan In alin. 1) Wakuu (1) Halimalt ut i THESES (2) Uwho comply wind 25 Secondary outcomes 2.5.1 Glycaemic variability and changes in diabetes medication GV was assessed from 48 hour continuous blood glucose manitaring (CGM, iPro 2 Medtronic; North Ryde. Australia and included SDcare mean amplitude of glycaemic excursions (MAGE, average of blood glucose excursions exceeding 1 SD of the man blood glu- cose values and continuous overal net glycaemic action (CONGA-1 And CONCA 4, 5D of differences between brvations 1 or 4 hour sl apart, respectively 22.14 An antiglycaemic Medication Effect Score (MES) based on medi cation potency and sage was used to assess changes in utilization of antiplycaemic agents including rolin Higher MES corresponds ta higher diabetes medication requirement Assessed ar 2 years (28) Dhenaiபடிப் பாபர் CUET 11 cualill Press lahko cery with distan) Analysis Entered data analyds in Entrada al 571 2.6 | Statistical analyses Primary analysis was by random-coefficient analysis, with data 29.med to be missing at random. Linear mixed-effects models that included fixed effects for each time-point and dist group gerent, and a det group by time point interaction were used to evaluate between group differences in outcomes. The restricted maximum likelihood, Inear mixed-effects model permits a variable number of olevations for participants and structured cowance sccounts for correlations between repeated measures over time. In accordance with an intention to treat principle, analyses included all available data from the 115 participants who commenced the study 2.5.2 Anthropometric data Height was measured using a stadiometer (SECA, Hamburg, Ger- mawl. badlywight using callarated dectronic Scales (Mercury AMZ1. Tokyo, Japan and waist circumference by tape measure post- tioned 3 cm above their crest. Body composition (at mas|FM and fat-free mass (FEMI was determined by whole body dual-energy FIGURE 1 Study flow claram were provided with key food packs wery second month and a 50 AUD voucher to subsidie purchase of key foods on every alter nate second month. Participants prepared/purchased their own food/ meals according to guidelines specific to their prescribed diets. Diet plans were individualized and energy matched with moderate (-20) restriction to facilitate weight loss (500-1000 kcal/day deficit: 1357- 862 LWILEY- TAY ET AL. of 25% (LC, 22; HC, 20; P = 0.69) and 34% achieved 210% weight reduction (LC, 12; HC, 9; P = 0.73). Baseline characteristics and exercise session attendance were com- pared by independent t-tests and y? tests for continuous and cate- gorical variables, respectively. Results are presented as estimated marginal means (95% confidence intervals, Cl) by linear mixed-effects model analysis using SPSS 20.0 for Windows (SPSS Inc.; Chicago, Illi- nois) unless otherwise stated. Changes from baseline to Week 104 are reported. All statistical tests were two-tailed using a signifi- cance level of p < 0.05. 2.7 | Sample size and power The study was designed to have 80% power to detect a previously reported 0.7% absolute difference in HbA1c (primary outcome) between diets,5,18,26 based on an anticipated -50% dropout rate, as typically observed in long-term diet and lifestyle interventions. 6,27,28 3.4 | Glycaemic control: HbA1c, glycaemic variability, anti-glycaemic medication effect score (MES) and insulin sensitivity HbA1c reductions were similar in both groups (-0.7 (-1.0, -0.5] %; P = 0.52) (Figure 3A). The LC group maintained greater reductions in diabetes medication requirements (antiglycaemic MES, LC, -0.5 (-0.6, -0.3], HC, -0.2 (-0.4, -0.02] units; P = 0.03) (Figure 3B). Over twice the number of LC participants had a 2 20% reduction in MES com- pared to HC participants (LC, 22; HC, 9). Greater reductions in GV (MAGE, SD Glucose, Glucose range, MODD, AUCTotal glucose per min, CONGA-1 and CONGA-4) occurred in the LC group compared to the HC group (p = 0.001-0.24) (Table 2, Figure 3). Differences persisted over 2 years and were statistically significant for CONGA-1 (LC, -0.4 (-0.6, -0.3], HC, 0.1 (-0.1, 0.2] mmol/L; P = 0.001) and CONGA-4 (LC, -0.9 [-1.3, -0.6], HC, -0.2 [-0.6, 0.1] mmol/L; P = 0.02) (Figure 3C,D). Fasting blood glucose and insulin markers (insulin, HOMA2-IR and HOMA2-%B) decreased, with no difference between groups (Table 2, P = 0.13). 3 | RESULTS 3.1 Baseline characteristics A total of 115 adults were randomized (LC, 57; HC, 58) (Figure 1). Baseline characteristics were well matched between groups (Tables 1 and 2). Most participants were using oral anti-glycaemic medications (LC, 48; HC,45); >75% were using metformin, 30% sulfonylureas and 10% exogenous insulin. Approximately two-thirds were using lipid- lowering medications and anti-hypertensives. Among the total, 53% of participants completed the study (LC, 33; HC, 28), with similar attrition and reasons for withdrawal between groups (P = 0.40) (Figure 1). 3.5 | CVD risk factors: blood pressure, lipids, endothelial function and CRP TAG decreased to a greater degree and HDL-C levels were main- tained with the LC compared to the HC (P < 0.004) (Table 2). Changes in non-HDL-C, total cholesterol, LDL-C, blood pressure and CRP did not differ between groups (P >0.44). Endothelial function (FMD) did not change in either group (p = 0.73). Concerning lipid- lowering medications, 5 participants reduced dosage (LC, 3; HC, 2) and 3 participants increased dosage (LC, 1; HC, 2). Concerning anti- hypertensive medications, 15 participants (LC, 10; HC, 5) reduced dosage and 5 participants (LC, 3; HC, 2) increased dosage. 3.2 | Dietary intake, physical activity and adherence measures 3.6 | Renal markers The two groups reported similar caloric intakes (P = 0.93). Dietary intakes were consistent with the prescribed diets (Table 3). Compared to the HC, the LC group reported lower intakes of carbohydrate, and higher intakes of protein and fat. Plasma and urinary biomarker data also reflected higher protein and lower carbohydrate intake in the LC group. The LC group experienced an initial three-fold greater increase in plasma -hydroxybutyrate levels compared to the HC group, with levels decreasing towards baseline over time (time x diet, P = 0.02) (Figure 51). The 24-hour urinary-urea data showed higher estimated protein intakes in the LC group (1.1-1.3 g/kg vs 1.0-1.1 g/kg, P<0.001). Compared to the HC group, the LC group experienced greater increases in 24-hour urinary-urea/creatinine excretion ratio, which remained higher over the study period (P = 0.001) (Table 1). Accelerometry data indicated that physical activity levels were similar between groups (P >0.37) (Table 1). Exercise session attendance was also similar between groups (LC, 56 + 24%; HC, 58 +24%; P = 0.80). eGFR levels remained in the normal to mildly depressed range in both groups. Comparable increases in SCr and reductions in eGFR and CrCl occurred in both groups (Table 2, P = 0.07). In 7 participants (LC, 4; HC, 3) albuminuria was moderately increased (AER 30- 300 mg/24 h) at baseline and was normalized and maintained in 4 participants (LC, 2; HC, 2). Albuminuria persisted in 2 participants (LC, 1; HC, 1) and 1 LC participant withdrew at Week 4 before study completion. All other participants who were normoalbuminuric at baseline remained so after 2 years. 3.7 | Adverse events 3.3 | Weight and body composition After 2 years, there were reductions in body weight (Figure 2), total FM and waist circumference, with no differences between groups (P >0.09 for all). Among completers, 69% maintained a weight loss There were no adverse event-related treatment discontinuations. A total of 21 participants (LC, 11; HC, 10) reported musculoskeletal ail- ments associated with exercise training. These participants continued the exercise program following recovery, although one participant from the HC group, who reported exacerbation of pre-existing fibro- myalgia secondary to resistance training (Week 64), withdrew from TABLE 1 Baseline participant characteristics and estimated marginal mean changes (95% CI) in body weight and composition, dietary biomarkers and physical activity after 2 years on a low- carbohydrate, high-unsaturated/low-saturated fat diet (LC) or an isocaloric high-carbohydrate, low-fat diet (HC) LC HC Mean difference in Variable Baseline Change Baseline Change change between groups P Value Participant characteristics Male/Female (n (%)] 37 (64) / 21 (36) 29 (51) / 28 (49) Age (years) ( 58 (56 to 60) 58 (56 to 60) Diabetes Duration (years) 6 (4 to 7) 8 (6 to 10) Body weight and composition Body-weight (kg) 101.7 (97.8 to 105.7) ) -6.8 (-8.8 to -4.7) 101.6 (97.6 to 105.6) -6.6 (-8.8 to -4.5) -0.1 (-3.1 to 2.8) 0.26 BMI (kg/m2 34.2 (33.1 to 35.3) -2.1 (-2.8 to -1.5) 35.1 (34.0 to 36.2) -23-3.0 to -1.6) 0.1 (-0.8 to 1.1) 0.33 Waist Circumference (cm) 112.4 (109.7 to 115.2) -7.9 (-10.0 to -5.7) 112.5 (109.8 to 115.3) -7.2 (-9.5 to -5.0) -0.6 (-3.7 to 2.5) 0.54 Total FFM (kg) 62.0 (59.1 to 64.8) () -2.2 (-2.8 to -1.6) 60.1 (57.2 to 62.9) -2.0 (-2.7 to -1.4) -0.2 (-1.0 to 0.7) ) 0.71 Total FM (kg) 39.8 (37.1 to 42.4) -4.3 (-6.2 to -2.4) 41.5 (38.8 to 44.2) -4.6(-6.6 to -2.7) 0.3 (-2.4 to 3.0) 0.09 FM:FFM ratio 0.7 (0.6 to 0.7) -0.1 (-0.1 to -0.02) 0.7 (0.7 to 0.8) -0.1 (-0.1 to -0.02) 0.01 (-0.04 to 0.1) 0.25 Dietary biomarkers 24 h Urinary Urea/ 39.9 (37.6 to 42.2) 4.2 (1.2 to 7.2) 38.7 (36.4 to 41.0) 0.8 (-2.5 to 4.0) 3.4 (-1.0 to 7.9) 0.001 Creatinine ratio Physical activity Mean activity count 188.9 (171.6 to 206,3) -12.3 (-34.2 to 9.7) 182.7 (165.2 to 200.2) -2.5 (-25.3 to 20.2) -9.8 (-41.4 to 21.8) 0.37 (counts/min) MVPA (min/day) 46.4 (41.4 to 51.5) 1.1 (-5.0 to 7.1) 44.0 (38.9 to 49.0) 0.6 (-5.7 to 7.0) 0.4 (-8.4 to 9.2) 0.62 MVPA (% of total 3.5 (3.2 to 3.9) -0.3 (-0.8 to 0.2) 3.4 (3.0 to 3.7) () -0.7 (-1.2 to -0.2) 0.4 (-0.3 to 1.1) 0.62 wear time) Abbreviations: LC, low-carbohydrate, high-unsaturated/low-saturated fat diet; HC, high-carbohydrate, low-fat diet; BMI, body mass index FM, fat mass; FFM, fat-free mass; MVPA, moderate to vigorous intensity physical activity. The following variables were square root transformed to attain normality before analyses: FFM; accelerometry data and urinary-urea/creatinine excretion ratio. Untransformed values are presented to facilitate interpretation. Baseline participant characteristics data are presented as mean (95% confidence interval). All other data are presented as estimated marginal means (95% confidence intervals) by linear mixed-effects model analysis, unless otherwise stated. Total analysed, n = 115 (LC, 58; HC, 57) for all data unless otherwise stated. P value refers to between-group differences over time (time x diet interaction) by linear mixed-effects model analysis. All baseline values were not significantly different between diet groups (P >0.05) by chi-square tests (categorical variables) and independent samples t-test (continuous variables). Total analysed, n = 114 (LC, 57; HC, 57) for 24-hour urinary urea/creatinine ratio data; no urine analysis was available at baseline for 1 participant in the LC group. Total analysed, n = 115 for accelerometry data; data from 2 participants (LC, 1; HC, 1) at 24 weeks, from 6 participants (LC, 4; HC, 2) at 52 weeks and from 1 participant in the LC group at 104 weeks that did not meet validity criteria were excluded from these analyses. d -WILEY 863 TABLE 2 Estimated marginal mean changes (95% CI) in glycaemic control, diabetes medication, cardiovascular disease risk and renal markers after 2 years on a low-carbohydrate, high-unsaturated/low- saturated fat diet (LC) or an isocaloric high-carbohydrate, low-fat diet (HC) – LC HC Baseline Change Baseline Change Mean difference in change between groups P Value b 0.13 0.18 7.8 (7.3 to 8.4) 8.4 (7.9 to 8.9) 8.4 (7.9 to 8.9) 4.8 4.5 to 5.2) 14.0 (13.1 to 14.9) 9.1 (8.3 to 10.0) 1.9 (1.6 to 2.1) 0.3 (-0.4, 1.0) -0.7(-1.4 to -0.02) -0.7 (-1.4, -0.02) 0.1 (-0.5 to 0.6) -2.0 (-3.1 to -0.8) -2.1 (-3.1 to -1.2) -0.4 (-0.7 to -0.1) 8.4 (7.8 to 9.0) 8.7 (8.2 to 9.1) 8.6 (8.1 to 9.1) 4.8 (4.5 to 5.2) 14.3 (13.4 to 15.2) 9.5 (8.7 to 10.3) 2.1 (1.8 to 2.3) -0.4 (-1.1 to 0.4) -0.6 (-1.4 to 0.1) -0.6 (-1.3 to 0.1) -0.3 (-0.8 to 0.3) ) -0.6 (-1.8 to 0.5) -0.6 (-1.6 to 0.4) -0.3(-0.6 to -0.004) 0.7(-0.3 to 1.7) -0.1 (-1.1 to 0.9) -0.1 (-1.1 to 0.9) 0.3 (-0.4 to 1.1) -1.3 (-2.9 to 0.3) -1.6 (-2.9 to -0.2) -0.1 (-0.5 to 0.3) 0.18 0.69 0.23 0.24 0.16 0.76 0.44 0.85 Variable Glycemic control Fasting glucose (mmol/L) Mean glucose (mmol/L) AUCTotal glucose per min (mmol/L) Minimum glucose (mmol/L) Maximum glucose (mmol/L) Glucose range (mmol/L) MODD (mmol/L) CVD risk markers SBP (mmHg) DBP (mmHg) Total Cholesterol (mmol/L) LDL-C (mmol/L) HDL-C (mmol/L) TG (mmol/L) Non-HDL-C (mmol/L) FMD (%) Fasting insulin (mU/L)* HOMA2-IR HOMA2-%B’ CRP (mg/L) Diabetes medications Antiglycaemic MES MES reduction 220% (n (%)] MES reduction 250% (n (%) h Renal markers Serum creatinine (umol/L) Urinary albumin (mg/L) AER (mg/24 h) ) 130.4 (126.9 to 133.8) 79.9 (77.4 to 82.4) 4.5 (4.2 to 4.7) 2.5 (2.3 to 2.8) ( 1.2 (1.1 to 1.3) 1.6 (1.5 to 1.8) ( 3.3 (3.0 to 3.5) 5.6 (4.8 to 6.5) 15.9 (13.8 to 18.1) 1.9 (1.7 to 2.2) 64.7 (55.8 to 73.5) 2.8 (2.2 to 3.4) ) -2.0(-5.9 to 1.8) -1.2-3.6 to 1.2) 0.2 (-0.1 to 0.6) 0.2 (-0.1 to 0.5) 0.02 (-0.05, 0.1) -0.1 (-0.3 to 0.2) 0.2 (-0.2 to 0.5) -0.5(-1.5 to 0.5) -2.6 (-4.6 to -0.7) -0.3 -0.5 to -0.1) -5.6 (-11.5 to 0.2) -0.6 (-1.1 to -0.03) 132.6 (129.1 to 136.0) 80.8 (78.2 to 83.3) 4.3 (4.0 to 4.6) 2.4 (2.2 to 2.6) ( 1.3 (1.2 to 1.3) 1.4 (1.3 to 1.6) 3.0 (2.8 to 3.3) ) 5.4 (4.5 to 6.2) 15.9 (13.7 to 18.1) 2.0 (1.7 to 2.2) ) 60.8 (51.7 to 69.9) 2.8 (2.2 to 3.5) -3.2 (-7.3 to 0.9) -2.0(-4.5 to 0.5) 0.1 (-0.3 to 0.4) 0.1 (-0.2 to 0.4) -0.1 (-0.1 to 0.01) 0.1 (-0.2 to 0.3) 0.1 (-0.2 to 0.5) -0.4 (-1.4, 0.7) -1.8 (-3.9 to 0.2) -0.2 (-0.5 to 0.03) -5.8 (-12.0 to 0.3) ( -0.8 (-1.4 to -0.2) 1.1-4.5 to 6.8) 0.8 (-2.7 to 4.2) 0.2 (-0.3 to 0.7) 0.1 (-0.3 to 0.5) 0.1 (-0.02 to 0.2) -0.2 (-0.5 to 0.2) 0.04 (-0.4 to 0.5) -0.1 (-1.5 to 1.3) -0.8 (-3.6 to 2.0) -0.1 (-0.4 to 0.3) 0.2 (-8.3 to 8.7) 0.2 (-0.6 to 1.0) 0.85 0.004 0.001 0.51 0.73 0.72 0.88 0.28 0.74 1.3 (1.0 to 1.5) 1.1 (0.9 to 1.4) -0.2 (-0.5 to 0.04) -0.5 (-0.6 to -0.3) 22 (38%) 12 (21%) -0.2 (-0.4 to -0.02) 9 (16%) 8 (14%) 0.03 0.04 1.0 69 (65 to 72) 3.3 (1.1 to 5.4) 6 (2 to 11) 3 (1 to 5) -0.9 (-2.8 to 1.1) 31-6 to 11) 71 (67 to 75) 2.4 (0.3 to 4.5) 5(1 to 9) 3 (0.3 to 5) -0.04 (-2.1 to 2.0) 0.3 (-9 to 9) 0.1 (-3 to 3) -0.8 (-3.7 to 2.0) 2(-10 to 15) 0.19 0.14 0.38 (Continues) b TABLE 2 (Continued) LC HC Mean difference in Variable Baseline Change Baseline Change change between groups P Value eGFR- CKD-EPI (ml/min/1.73m2. 96 (93 to 99) -4 (-6 to -2) 91 (88 to 95) -3 (-5 to -1) -1 (-4 to 2) 0.32 CrCl-Cockcroft-Gault (ml/min/kn 82 77 to 87) -8 (-10 to -5) 77 (72 to 82) -8 (-11 to -5) 0.4 (-3 to 4) 0.33 CrCl-Salazar-Corcoran (ml/min). 127 (119 to 134) -12 (-16 to -8) 120 (112 to 127) -134-17 to -9) 1 (-5 to 7) 0.07 Abbreviations: LC, low-carbohydrate, high-unsaturated/low-saturated fat diet; HC, high-carbohydrate, low-fat diet: AUCTotal glucose per mine total area under the curve for blood glucose standardized by valid wear time; MODD, mean of daily differences between paired blood glucose values during successive 24-hour periods: SBP, systolic blood pressure: DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL- C. high-density lipoprotein cholesterol: TG, triglycerides: Non-HDL-C, non-high-density lipoprotein cholesterol: FMD, flow-mediated dilatation; HOMA-IR, homeostasis model of assessment index 2-insulin resistance; HOMA2-%B, homeostasis model of assessment index 2- cell function; CRP, C-reactive protein; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate; CKD-EPI, chronic kidney disease epidemiology collaboration; CrCl, creatinine clearance. To convert mmol/L to mg/dL, multiply by 18 (for glucose), by 38.7 (for cholesterol) and by 88.6 (for triglycerides). To convert umol/L to mg/dl, multiply by 0.0 (for serum creatinine). The following variables were transformed to attain normality before analyses: HbA1c, AUCTotal plucci per min mean and fasting glucose, AER and urinary albumin (reciprocal transformation); insulin, HOMA2-IR and HOMA2-XB (square root transformation); TG, FMD, minimum and maximum glucose, SD Clucose glucose range, MAGE, CONGA-1, CONGA-4, MODD, CRP and MES, (logarithmic transformation). Untransformed values are presented to facilitate interpretation. All data are presented as estimated marginal means (95% confidence intervals) by linear mixed-effects model analysis, unless otherwise stated. Total analysed, n – 115 (LC, 58; HC, 57) for all data unless otherwise stated. P value refers to between-group differences over time (time x diet interaction) by linear mixed-effects model analysis. Total analysed, n = 113 (LC, 57; HC, 56) that met the criteria for 48-hour valid continuous glucose monitoring (CGM) data collection to calculate comparisons between successive days. Non-HDL-C was calculated as the difference between total cholesterol and HDL cholesterol concentration. * Total analysed, n = 115 for FMD data; outlying data from 1 LC participant at base
(Visited 2 times, 1 visits today)