1. What is the purpose of the article?
2. What type of study design was used in this article?
3. Describe briefly the two groups utilized in the study.Identify which is the control group and which is the treatmentgroup.
4. What were the inclusion/exclusion criteria for thestudy?
5. List Several Advantages and Disadvantages (in general) for thistype of study design?
6. How was the outcome defined in this study?
7. What is the exposure in this study?
8. What results were reported in the article?
9. Is there anything else that stands out in the article that youthink should be mentioned?
Transcribed Image Text from this Question
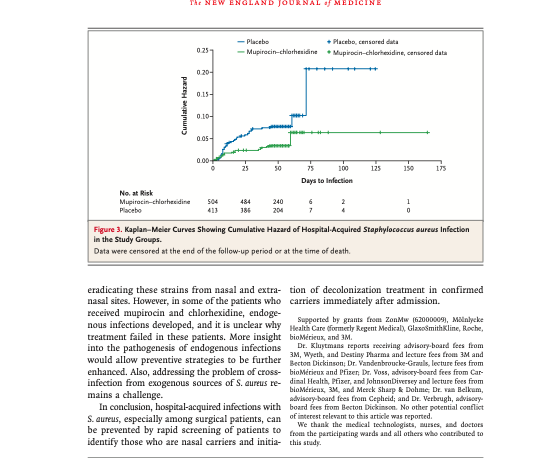
ABSTRACT RESULTS BACKGROUND Nasal carriers of Staphylococcus aureus are at increased risk for health care-associated from the Department of Medical Micro- infections with this organism. Decolonization of nasal and extranasal sites on hos- biology and Infectious Diseases, Eras- pital admission may reduce this risk. mus University Medical Center, Rotter- dam (L.G.M.B., H.F.L.W., A.B., H.A.V., M.C.V.); the Laboratory of Microbiology METHODS and Infection Control, Amphia Hospital, In a randomized, double-blind, placebo-controlled, multicenter trial, we assessed of Medical Microbiology and Infection whether rapid identification of S. aureus nasal carriers by means of a real-time poly- Control, VU Medical Center, Amsterdam merase-chain-reaction (PCR) assay, followed by treatment with mupirocin nasal J.A.J.W.K., C.M.J.E.G., R.R.); the De- ointment and chlorhexidine soap, reduces the risk of hospital-associated S. aureus A.LA.B.) and the Julius Center for Health infection. Sciences and Primary Care (1.T.), Univer- sity Medical Center, Utrecht; the Depart- ment of Medical Microbiology and In- fectious Diseases, Canisius-Wilhelmina From October 2005 through June 2007, a total of 6771 patients were screened on Hospital (A.V.), and the Center for Ortho- admission. A total of 1270 nasal swabs from 1251 patients were positive for S. aureus. pedic Surgery, Sint-Maartenskliniek (A.V.), We enrolled 917 of these patients in the intention-to-treat analysis, of whom 808 Nijmegen – all in the Netherlands, and (88.1%) underwent a surgical procedure. All the S. aureus strains identified on PCR Hanoi, Vietnam (H.E.L.W.). Address re- assay were susceptible to methicillin and mupirocin. The rate of S. aureus infection print requests to Dr. Bode at Erasmus was 3.4% (17 of 504 patients) in the mupirocin-chlorhexidine group, as compared of Medical Microbiology and Infectious with 7.7% (32 of 413 patients) in the placebo group (relative risk of infection, 0.42; Diseases, ‘s Gravendijkwal 230, 3015 CE 95% confidence interval [CI], 0.23 to 0.75). The effect of mupirocin-chlorhexidine Rotterdam, the Netherlands, or at l.bodem treatment was most pronounced for deep surgical-site infections (relative risk, 0.21; 95% CI, 0.07 to 0.62). There was no significant difference in all-cause in-hospital mor- N Engl J Med 2010;362:9-17. tality between the two groups. The time to the onset of nosocomial infection was copyright © 2010 Massachusetts Medical Society shorter in the placebo group than in the mupirocin-chlorhexidine group (P=0.005). CONCLUSIONS The number of surgical-site S. aureus infections acquired in the hospital can be re- duced by rapid screening and decolonizing of nasal carriers of S. aureus on admis- sion. (Current Controlled Trials number, ISRCTN56186788.) erasmusmc.nl. VAL N ASAL CARRIERS OF HIGH NUMBERS OF METHODS Staphylococcus aureus organisms have a risk of health care-associated infection with STUDY DESIGN this microorganism that is three to six times the The study was a randomized, double-blind, place- risk among noncarriers and low-level carriers. 1-3 bo-controlled clinical trial, conducted at three uni- More than 80% of health care-associated S. aureus versity hospitals and two general hospitals in the infections are endogenous. Netherlands. From October 2005 through June Intranasal application of mupirocin has been 2007, we screened patients who were admitted to shown to be effective for the decolonization of this the departments of surgery and internal medicine, microbe and the prevention of invasive S. aureus where the risk for S. aureus infection is high. The infections in patients receiving long-term dialysis primary outcome of the trial was the cumulative treatment. However, in other nonsurgical pa- incidence of hospital-associated S.aureus infections. tients, mupirocin had no effect on the rate of Secondary outcome measures included all-cause health care-associated S. aureus infections. Mupi- in-hospital mortality, duration of hospitalization, rocin nasal ointment was reported to be effective and time from admission to the onset of health in preventing surgical-site infections in surgical care-associated S. aureus infections. The institu- patients, but this study used a historical control tional ethics committee at each center approved group. Two randomized, controlled trials failed the protocol. Oral informed consent was obtained to show a reduction in rates of surgical site infec at the time of screening. Once a patient was ran- tion in orthopedic and general-surgery popula- domly assigned to decolonization with either mupi- tions, although a subgroup analysis in one of rocin-chlorhexidine or placebo, written informed these studies suggested that intranasal mupiro- consent was obtained. The manufacturers of the cin may be effective in preventing health care products provided the trial medications and pla- associated S. aureus infections in carriers of this cebo at no cost but did not influence the study de- organism.13.14 sign, data collection, analysis, writing, or decision Several explanations have been offered for these to submit the results for publication. failures. In some studies, failure of decolonization may have been due to the timing of treatment. INCLUSION AND EXCLUSION CRITERIA If decolonization is started only after the results Patients were screened by trained nursing staff for of screening cultures become available, health nasal carriage of S. aureus either immediately on care-associated infections may already be incu- admission or during the week before admission, bating and may therefore be difficult to prevent with decolonization therapy begun at the time of With the development of rapid screening tests for admission. The inclusion criterion for screening S.aureus, the carrier status can be assessed within was the expectation that a patient would remain hours after admission. 5-7 Another explanation hospitalized for at least 4 days in one of the par- could be that nasal carriers of S. aureus are also ticipating departments internal medicine, cardio- colonized at extranasal sites. It is unlikely that thoracic surgery, vascular surgery, orthopedics, nasal application of mupirocin will directly affect gastrointestinal surgery, or general surgery). The these sites. However, decolonization of the skin exclusion criterion for screening was an age of less can be achieved by washing with disinfecting soap, than 18 years. Inclusion criteria for randomiza- such as chlorhexidine gluconate products.” tion were nasal carriage of S. aureus as determined We conducted a randomized, double-blind, by real-time PCR and the ability to start the inter- placebo-controlled, multicenter clinical trial in vention within 24 hours after the patient’s admis- which we rapidly identified nasal carriers of S.au- sion to a participating ward. The expected duration reus by real-time polymerase-chain-reaction (PCR) of hospitalization was estimated again immedi- assay on admission. In S. aureus carriers only, we ately before randomization and had to be at least assessed whether decolonization of the nostrils 4 days. Exclusion criteria for randomization were with mupirocin ointment and of the skin with the presence of active infection with S. aureus at chlorhexidine gluconate soap could prevent hos- the time of randomization, known allergy to mupi- pital-associated infections with S. aureus. rocin or chlorhexidine, pregnancy, breast-feeding. use of mupirocin in the preceding 4 weeks, and colonization and to determine whether the infec- the presence of a nasal foreign body. tion was health care-associated according to cri- teria established by the Centers for Disease Con- RANDOMIZATION trol and Prevention. The results of all clinical Patients were randomly assigned in a 1:1 ratio to cultures performed during the follow-up period either active treatment with mupirocin ointment were also documented. In surgical patients, stan- 24% (Bactroban, GlaxoSmithKline) in combination dard presurgical prophylactic antimicrobial ther- with chlorhexidine gluconate soap, 40 mg per mil- apy was given according to the local hospital liliter (Hibiscrub, Molnlycke), or placebo ointment guidelines. in combination with placebo soap. Placebo soap and ointment were identical to the active treatment MICROBIOLOGIC RESULTS except for the active ingredients. A single list of To screen patients for S. aureus carriage, a dry, ster- random numbers with a permuted-block design ile rayon swab (Becton Dickinson) was rotated was generated by an independent statistician and four times in each nostril. The swab was placed in distributed to all participating centers. 100 ml of saline and centrifuged. Part of the sam- ple was processed for real-time PCR, and part was ENROLLMENT AND FOLLOW-UP inoculated onto a blood agar plate, to allow nasal Patients were asked to participate by a member of and infecting strains to be compared in order to the trial team. Immediately after providing written determine whether an infection was endogenous informed consent, the patient was assigned to ei- or exogenous. Culture results were not used to 25- ther the active treatment or the placebo according sess eligibility for randomization Cultured strains to the randomization list, and the first dose of were genotyped by means of pulsed-field gel elec- nasal ointment was applied. Nasal ointment was trophoresis to compare them on a clonal level, applied twice daily, and the soap was used daily and results were evaluated according to standard for a total body wash. The duration of the study criteria. Further details on the microbiologic treatment was 5 days, irrespective of the timing testing are included in the Supplementary Appen- of any interventions. Patients who were still hos- dix, available with the full text of this article at pitalized after 3 weeks and those still hospital- NEJM.org. ized after 6 weeks received a second and third course of the same trial medication, respectively. STATISTICAL ANALYSIS The follow-up period for S. aureus infection was on the basis of previous studies, the estimated the first 6 weeks after discharge. We defined the cumulative incidence of health care-associated time to infection as the time from randomization S. aureus infections in carriers of S. aureus is 6%. to the onset of infection. Data were censored when We originally planned to enroll 1800 subjects for follow-up for S. aureus infection ended or at the randomization to achieve a power of 80% with a time of death. Time periods for the end points of two-tailed type I error rate of 0.05 and a reduc- length of hospital stay and mortality were mea- tion of 50% in health care-associated S. aureus sured from the primary admission until 6 weeks infections. After 860 patients had been enrolled, after discharge from the primary admission. If a perceived change in the cumulative incidence of a patient was readmitted to the hospital within serious S.aureus infections was reported in one of 6 weeks after discharge from the primary admis- the participating centers. On request, the institu- sion, the number of hospital days during the sub- tional ethics committee at each center approved sequent admission were included in the calcula- a sequential analysis of the accumulated data set tion of length of stay by an independent statistician. Analysis of the data Patients were monitored for hospital-acquired from the first 400 patients showed that the upper S.aureus infection by means of microbiologie cul- boundary was crossed; thus, there was sufficient tures. Attending physicians were encouraged to evidence to conclude that the difference in out- obtain culture samples if infection was suspected. comes between the two study groups was sig- If a culture grew S. aureus, the patient’s medical nificant. At the time of the sequential analysis, record was reviewed to distinguish infection from additional patients had already undergone ran- The NEW ENGLAND JOURNAL OF MEDICINE PREVENTING SURGICAL-SITE INFECTIONS IN S. AUREUS CARRIERS 6771 Patients were screened for nasal Son POR 1251 Tested positive for Son PCR 353 Were escluded 146 Dedined to participate 140 Did not meet inclusion criteria 47 Had other reasons 20 et exclusion criteria 918 Underwent randomization SOS Received mupirocin chlorhexidine 413 Received placebo Table 1. Baseline Characteristics of the 917 Study Patients. Mupirocin-Chlorhexidine Placebo Characteristic IN-504) IN413) P Value Mean (SD) agey 61.8113,9 62.8+13.3 0.25 Male sex-no: 156) 331 [65.7) 251 (60.81 0.13 Hospital service no. (%) Surgery 441 [87.5) 367 (88.91 0.53 Internal medicine 63 [12.5) 46 (11.1) 0.53 Admission during month before current admission 86/503 (17.1) 67/411 (16.31 0.76 no/total no. (%) McCabe score at admission Median 1 1 Interquartile range 1-2 1-2 Underlying disorder-no/total no. (%) Diabetes mellitus type 1 or 2 112/503 122.3 ) 71/412 (17.2) 0.06 Disorder requiring continuous ambulatory peritoneal 7/504 (1.4) 4/413 [1.01 0.57 dialysis Renal insufficiency 24/504 (4.8) 23/413 15.6) 0.57 Immunodeficiency 19/504 (3.8) () 31/413 [7.5) 0.01 Liver-function disorder 25/504 15.01 22/413 (5.3) 0.80 Malignant condition 63/504 [12.5) 46/413 [11.2) 0.54 Skin disease 52/501 [1024 58/408 (142) 0.06 Antibiotic therapy-no/total no. (%) At time of admission 17/504 3.4) 16/413 3.9) 0.69 During month before admission 41/500 (8.2) 28/403 (6.9 0.46 * We used the McCabe score, as modified by Doern et al.,” to classify the severity of the underlying disease as follows: 1. nonfatat 2. possibly fatal; 3, ultimately fatal, and 4. rapidly fatal. Details concerning the definition of immunodeficiency are available in the Methods section of the Supplementary Appendix wwhdrew consent 501 Were included in the analysis 413 Were included in the analysis Figure 1. Study Enrollment and Randomization Of the 918 patients who underwent randomization, I was inadvertently assigned to treatment with mupirocin-chlor hexidine despite a nasal swab that was negative for Staphylococcus aureus on polymerase-chain-reaction (PCR) assay domization, and the data for these patients were between the two treatment groups is significant added to the final analysis. The analysis was strat- (P=0.008). (A detailed description of this analy- ified according to center and was based on the sis is available in the Supplementary Appendix.) intention-to-treat principle. The cumulative incidence of health care-asso- ciated S. aureus infection was significantly lower RESULTS in the mupirocin-chlorhexidine group than in the placebo group (Table 2). Among the 917 patients STUDY POPULATION who underwent randomization, 49 had hospital- In total, 6771 patients were screened for the pres- acquired S. aureus infections: 17 (3.4%) in the ence of S. aureus in the nasal passages. Results mupirocin-chlorhexidine group and 32 (7.7%) in were positive for S. aureus on real-time PCR in the placebo group (relative risk with mupirocin- 1270 samples (18.8%) obtained from 1251 patients. chlorhexidine, 0.42; 95% confidence interval [CI), Of the 918 patients who underwent randomiza- 0.23 to 0.75). In the sequential analysis we cor- tion, 1 withdrew consent and was excluded from rected for the imbalance between the groups with the analysis (Fig. 1). Six patients in the mupirocin- respect to the proportion of immunocompromised chlorhexidine group and 11 patients in the pla- patients, but this did not affect the outcome. The cebo group received a second or third course of number of patients who would need to be screened treatment. Table 1 shows the baseline character- and the number of S. aureus carriers who would istics of the patients. need to be treated to prevent one hospital-acquired S. aureus infection were 250 and 23, respectively. STUDY OUTCOMES Logistic regression analysis showed no signifi- Figure 2 shows the results of the sequential cant difference in the primary outcome between analysis of the cumulative data. The data cross surgical and nonsurgical patients. The number of the upper boundary, indicating that there is suf nonsurgical patients was small (109 of the 917 ficient evidence that the difference in outcome patients included in the analysis (11.9%]). Out- comes for the surgical and nonsurgical patients log-rank test). Figure 3 shows the cumulative haz- are presented separately in Table A in the Supple- ard of hospital-acquired S. aureus infection in both mentary Appendix. Deep surgical-site infections study groups. were most frequent (Table 2). Among the surgi- The mean duration of hospitalization was sig- cal patients, this type of infection occurred sig- nificantly shorter in the mupirocin-chlorhexidine nificantly less frequently in the 441 patients in group than in the placebo group (crude estimate, the mupirocin-chlorhexidine group than in the 12.2 vs. 14.0 days; P=0.04). Crude estimates of the 367 patients in the placebo group (4 infections median duration of hospitalization were 9 days (0.9%) vs. 16 [4.4%); relative risk, 0.21; 95% CI, in the mupirocin-chlorhexidine group and 10 days 0.07 to 0.62). in the placebo group (P=0.08). All-cause in-hos- Of the 49 strains causing infection, 47 were pital mortality did not differ significantly between available for molecular typing to determine wheth- the groups (2.6% in the mupirocin-chlorhexidine er the infection had an endogenous or exogenous group and 3.1% in the placebo group; relative risk source. The results of molecular typing are shown with mupirocin-chlorhexidine, 0.82; 95% CI 0.37 in Table 2. to 1.78). Of the 13 patients in the mupirocin- The time to infection with S. aureus was signifi- chlorhexidine group who died, 1 had a hospital- cantly shorter in the placebo group than in the acquired S. aureus infection. Of the 13 patients in mupirocin-chlorhexidine group (P=0.005 by the the placebo group who died, 3 had a hospital-
(Visited 3 times, 1 visits today)