Question: A protease similar to chymotrypsin was found to have 2 methionine residues in its binding pocket….
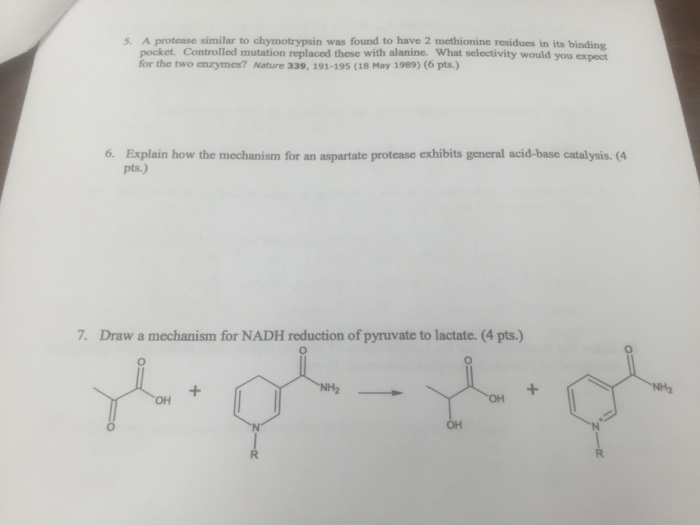
Show transcribed image text A protease similar to chymotrypsin was found to have 2 methionine residues in its binding pocket. Controlled mutation replaced these with alanine What selectivity would you expect for the two enzymes? Nature 339. 191-195 (18 May 1989) 6. Explain how the mechanism for an aspartate protease exhibits general acid-base catalysis. Draw a mechanism for NADH reduction of pyruvate to lactate.
A protease similar to chymotrypsin was found to have 2 methionine residues in its binding pocket. Controlled mutation replaced these with alanine What selectivity would you expect for the two enzymes? Nature 339. 191-195 (18 May 1989) 6. Explain how the mechanism for an aspartate protease exhibits general acid-base catalysis. Draw a mechanism for NADH reduction of pyruvate to lactate.



