Transcribed Image Text from this Question
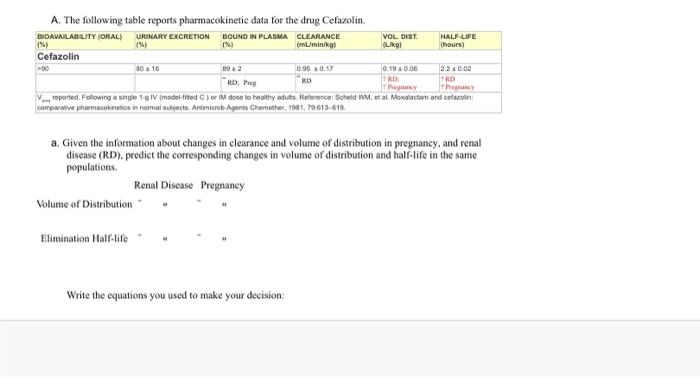
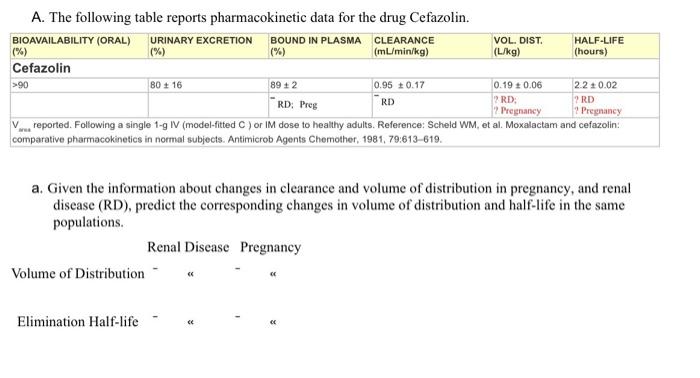
A. The following table reports pharmacokinetic data for the drug Cefazolin. BIOAVAILABILITY (ORAL) URINARY EXCRETION BOUND IN PLASMA CLEARANCE VOL DIST HALF-LIFE 1%) 096 (mL/min/kg LO Thours) Cefazolin 10 10 0.95 10.17 0,19 20.06 RD RD RD V reported. Following a single model fred or doce to healthy nuts. Reference Scheld WM, et al. Moxalacton and clarin compartive pharmacokinetics in nommal subjects. Animicrob pents Chemother. 190.79.613-610 a. Given the information about changes in clearance and volume of distribution in pregnancy, and renal disease (RD), predict the corresponding changes in volume of distribution and half-life in the same populations. Renal Disease Pregnancy Volume of Distribution Elimination Half-life Write the equations you used to make your decision: Write the equations you used to make your decision: b. Calculate total body clearance for a normal adult (70 kg) in ml./min and Uhr: c. Calculate renal clearance for a normal adult (70 kg) in ml/min and Uhr: d. Calculate renal filtration clearance for cefazolin (assume normal adult) e. Does the drug cefazolin undergo renal secretion? Why? Carter the rate of evention in mo/hr when the nlama incentration of cofeelinie ? mall A. The following table reports pharmacokinetic data for the drug Cefazolin. BIOAVAILABILITY (ORAL) URINARY EXCRETION BOUND IN PLASMA CLEARANCE VOL. DIST. HALF-LIFE (%) (mL/min/kg) (L/kg) (hours) Cefazolin >90 80 +16 892 0.95 +0.17 0.19 0.06 2.2 10.02 RD RD: RD: Preg ? RD Pregnancy Pregnancy V reported. Following a single 1-g IV (model-fitted) or IM dose to healthy adults. Reference: Scheld WM, et al. Moxalactam and cefazolin: comparative pharmacokinetics in normal subjects. Antimicrob Agents Chemother, 1981, 79:613-619. a. Given the information about changes in clearance and volume of distribution in pregnancy, and renal disease (RD), predict the corresponding changes in volume of distribution and half-life in the same populations. Renal Disease Pregnancy Volume of Distribution Elimination Half-life b. Calculate total body clearance for a normal adult (70 kg) in mL/min and L/hr: c. Calculate renal clearance for a normal adult (70 kg) in mL/min and L/hr: d. Calculate renal filtration clearance for cefazolin (assume normal adult) e. Does the drug cefazolin undergo renal secretion? Why? f Calculate the rate of excretion in ma/hr when the nlacma concentration of cefazolin is 02 molt
(Visited 5 times, 1 visits today)