Transcribed Image Text from this Question
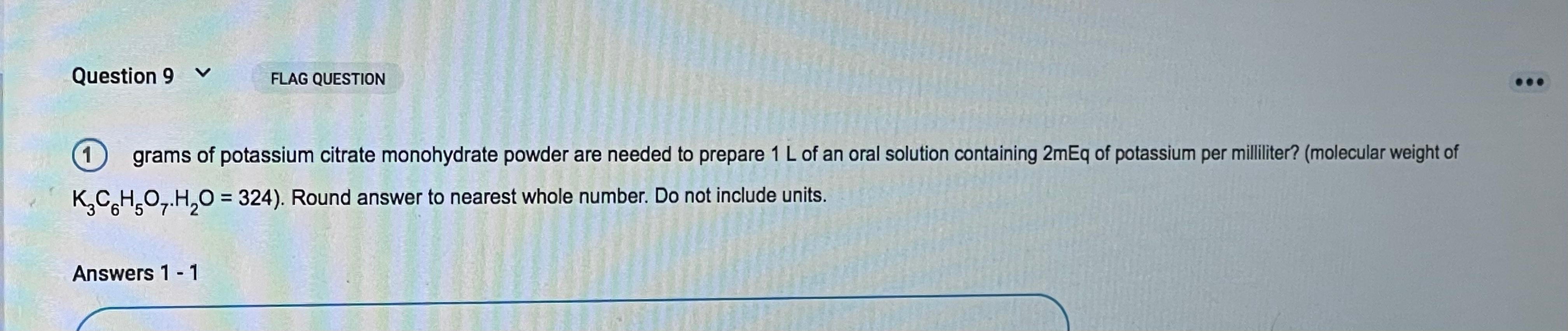
Question 9 V FLAG QUESTION 1 grams of potassium citrate monohydrate powder are needed to prepare 1 L of an oral solution containing 2mEq of potassium per milliliter? (molecular weight of K_CHO_.H2O = 324). Round answer to nearest whole number. Do not include units. Answers 1 – 1
(Visited 4 times, 1 visits today)
Related posts:
- Question: Question 1 FLAG QUESTION Is The Concentration, In Milligrams Per Milliliter, Of A Solution Containing 23.5 Mg Of Sodium Chloride Per Milliliter. Round Answer To The Nearest Whole Number. Do Not Include Units. (MW Of NaCl Is 58,5) Answers 1 – 1 1.
- Question: Question 2 FLAG QUESTION . A Solution Contains 5% Dextrose And 0.45% Sodium Chloride. The Osmolarity Of The Solution Is Molecular Weight Of Dextrose = 180, Molecular Weight Of NaCl = 58.5. Round Answer To The Nearest Tenth Do Not Include Units. Answers 1-1
- Question: Question 2 FLAG QUESTION A Solution Contains 5% Dextrose And 0.45% Sodium Chloride. The Osmolarity Of The Solution Is 1 Molecular Weight Of Dextrose = 180, Molecular Weight Of NaCl = 58.5. Round Answer To The Nearest Tenth. Do Not Include Units. Answers 1-1
- Question: Question 3 FLAG QUESTION Trace Electrolytes Additive (Tracelyte) Contains 0.25 MEq Of Calcium Per Milliliter. If 20 ML Of Tracelyte Is Included In 1 L Of TPN Solution, This Would Be Equivalent To 1 Milligrams Of Calcium Chloride (molecular Weight Of CaCl, = 111). Round Answer To The Nearest Tenth. Do Not Include Units.
- Question: Rx: AgNO3 0.25 G KNO3 0.12 G Water Ad Q.s. 45 ML Make Isotonic Solution. How Many Milliliters Of (a) Water And (b) Normal Saline Solution Is Needed To Make The Solution Isotonic? AgNO3 (m.w. 170; E-value 0.33), KNO3 (m.w. 101; E-value 0.58). A. 16.90 ML H2O; 28.10 ML Normal Saline B. 21.75 ML H2O; 23.25 ML Normal Saline C. 28.10 ML H2O; 16.90 ML Normal …
- Question: Question 17 FLAG QUESTION … … How Much 1 : 25 Solution And 1:500 Solution Should You Mix To Make 1 L Of A 1 : 250 Soaking Solution? Answers Are Rounded To The Nearest Whole Number. Answers A-D A 750 ML Of The 1 : 25 Solution And 250 ML Of The 1: 500 Solution B 250 ML Of The 1: 25 Solution And 750 Of The 1 : 500 Solution с 53 ML Of The 1 : 25 Solution …
- Question: Question 8 FLAG QUESTION K-TAB Tablets Contain 20 MEq Of Potassium In The Form Of Potassium Chloride 1 Is Milligrams Of Potassium Chloride In Each Tablet. Round To The Nearest Whole Number. Do Not Include Units. (MW Of KCl Is 74.5) Answers 1-1
- Question: Question 21 V FLAG QUESTION 1 Is The Osmolar Strength Of A Potassium Gluconate (C6H11K07: MW = 234) Solution Containing 2.5 Milliequivalents In Each 100 ML. Round Answer To The Nearest Whole Number. Do Not Include Units. Answers 1-1
- Question: Question 5 L FLAG QUESTION 1 Grams Of Sodium Chloride (MW Of NaCl Is 58.5) Should Be Used To Prepare 220 ML Of A Sodium Chloride Solution Containing 3 MEq Na Per ML. Round To The Nearest Tenth. Do Not Include Units.
- Question: 1. Order: Vancomycin 0.5 G IV Q.12h Supply: Vancocin 150 Mg Per ML True Or False: The Nurse Will Administer 2.33 ML ≈≈ 2 ML 2. Order: Penicillin G Potassium 400000 Units IM Q.4h Reconstitution: A) 18.2 ML Per 250000 Units Yields _____________ B) 8.2 ML Per 500000 Units Yields ______________ C) 3.2 ML Per 1000000 Units Yields _____________ D) 0.2 …