Transcribed Image Text from this Question
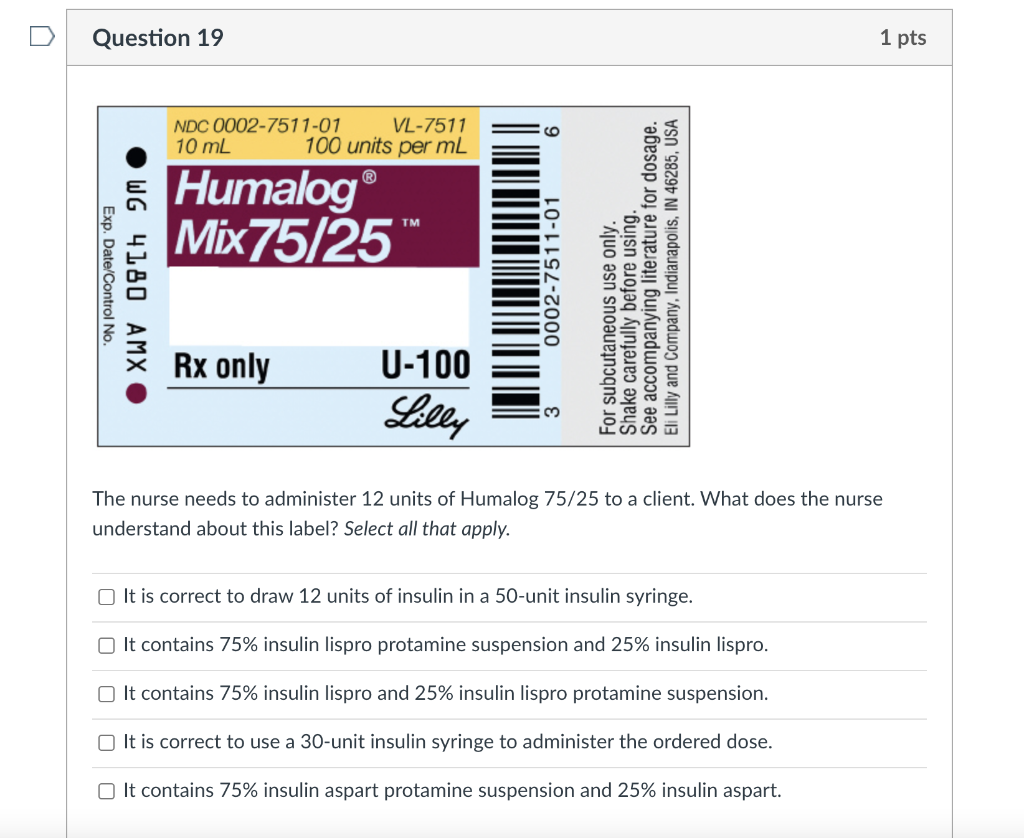
U-100 NDC 0169-1833-11 10 mL 100 units/mL Novoline Exp. Date/Control: “R Regular, Human Insulin Injection (recombinant DNA origin) USP (01) 103 0169 1833 111 Novo Nordisk • Important: see insert • Keep in a cold place • Avoid freezing Novo Nordisk Inc. Princeton, NJ 08540 1-800-727-6500 Manufactured by Novo Nordisk AVS DK-2880 Bagsvaerd Denmark What is correct regarding the drug label shown? Select all that apply. The Novolin R is the brand name. The dosage strength of the drug is 10 mL. The large capital letter R indicates human insulin. The concentration is 100 units of insulin per milliliter. o Only C and D Question 19 1 pts NDC 0002-7511-01 VL-7511 10 mL 100 units per mL 9 Humalog Mix75/25 TM Exp. Date/Control No WG 4180 AMXO 0002-7511-01 For subcutaneous use only. Shake carefully before using. See accompanying literature for dosage. Eli Lilly and Company, Indianapolis, IN 46285, USA Rx only U-100 Lilly The nurse needs to administer 12 units of Humalog 75/25 to a client. What does the nurse understand about this label? Select all that apply. It is correct to draw 12 units of insulin in a 50-unit insulin syringe. It contains 75% insulin lispro protamine suspension and 25% insulin lispro. It contains 75% insulin lispro and 25% insulin lispro protamine suspension. It is correct to use a 30-unit insulin syringe to administer the ordered dose. It contains 75% insulin aspart protamine suspension and 25% insulin aspart.
(Visited 2 times, 1 visits today)